Essential Question 4: How can we deliver gene therapies for larger genes?
Share This Post

Each Thursday throughout the summer, CureGRIN will be sharing a blog post that summarizes one of the 10 “Essential Questions” that need to be answered in order to find cures and treatments for GRIN Disorder. These questions are a central component of our recently unveiled Research Roadmap titled, “Treat the Symptoms. Cure the Disease.” You can find links to all of these blog posts here.
Today, we focus on Essential Question 5: How can we deliver gene therapies for larger genes?
There are many types of gene therapy medicines and strategies. Researchers have some ideas on certain gene therapy medicines and strategies that could possibly be developed to treat GRIN and other GRI disorders, though no gene therapy medicines are currently available for GRIN or other GRI disorders. The ideal gene therapy would entail only one medicine per gene, regardless of the specific variant. One type of gene therapy strategy that could be developed is called “Knockdown and Replace.”
“Knockdown and Replace” gene therapy would be delivered through the central nervous system or directly into the brain and would have two instructions. Firstly, it would tell the gene to stop making proteins from both copies of the patient’s genes. Secondly, it would introduce and boost a synthetic copy of the gene. The result would be that some mutant proteins could still possibly get made, but they would be vastly outnumbered by the wildtype proteins.
This strategy of gene therapy could be delivered using Adeno-Associated Virus Vectors (AAVs). There are currently two FDA approved AAV gene therapies. An AAV therapy called Luxturna was approved for a rare inherited retinal dystrophy in 2017, and an AAV therapy called Zolgensma was approved for spinal muscular atrophy in 2019.
AAVs are essentially carriers that are altered from a naturally occurring virus into a delivery tool for gene therapy. The viral genetic material is replaced with new genetic material. Then, the AAV is used to deliver wildtype copies of a gene to the right tissues or organs in the body.
AAVs have a carrying capacity of about 4.7kb of nucleotides. The “instructions” for “Knockdown and Replace” gene therapy contain approximately 1.1kb of code, meaning that the maximum size of a gene that can fit is approximately 3.6kb. The GRIN1 and GRIA genes likely fit into AAV. As seen in the table below, some of the GRIN genes are too big to fit into this type of vector.
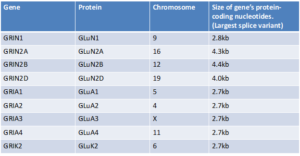
So, CureGRIN will be working with researchers to figure out how we can safely and effectively deliver gene therapies to patients with GRIN2A, GRIN2B, and GRIN2D Disorder. Some possible strategies to consider may include using dual AAVs or using other types of vectors such as adenovirus vectors and lentivirus vectors. We may also research additional gene therapy candidates like antisense oligonucleotides (ASOs), mRNA, and/or CRISPR. CureGRIN will partner with researchers and biotechnology companies to assess what the right strategy is for the delivery of gene therapy for GRIN2A, GRIN2B, and GRIN2D.
Read more Posts

How fast can we get to treatments and cures?
The more work we get done through this first round, the faster we’ll get to better treatments and cures.

